Abbott Laboratories is an American multinational medical devices and health care company with headquarters in Abbott Park, Illinois, United States. The company was founded by Chicago physician Wallace Calvin Abbott in 1888 to formulate known drugs; today, it sells medical devices, diagnostics, branded generic medicines and nutritional products. It split off its research-based pharmaceuticals business into AbbVie in 2013.
Abbott's products include Pedialyte, Similac, BinaxNOW, Ensure, Glucerna, ZonePerfect, FreeStyle Libre, i-STAT and MitraClip.
Products
Nutrition
Pediatric nutrition products manufactured by Abbott Laboratories include:
Similac
Pedialyte
PediaSure
Adult nutrition products manufactured by Abbott Laboratories include:
Ensure
Glucerna (Ensure Diabetes Care in India)
Juven
ZonePerfect
Diagnostics
Diagnostics products manufactured by Abbott include:
i-STAT (While intended for a human audience, the point of care analyzers also demonstrate utility for the veterinary profession and are marketed by Abaxis.)
Alinity
Architect
BinaxNOW
Medical devices
Continuous glucose monitors:
FreeStyle Libre
FreeStyle Libre 2 Sensor
FreeStyle Libre 3 Sensor
Cardiovascular devices manufactured by Abbott Laboratories include:
MitraClip
Confirm Rx
Amplatzer Piccolo Occluder
Heartmate
Xience
CARDIOMEMS
Gallant ICD
CentriMag
Aveir DR
Neuromodulation devices manufactured by Abbott Laboratories include:
BurstDR Technology
FlexBurst360 Technology
Proclaim DRG Neurostimulation System
Infinity Deep Brain Stimulation System
Proclaim XR Recharge-Free Spinal Cord Stimulator
NT2000IX Radiofrequency Generator
Proclaim Elite Recharge-Free SCS System
Prodigy MRI SCS System
Keeping your heart healthy with medical technologies that help you and your doctor better manage your health.
Abbott Cardiovascular
5050 Nathan Lane North
Plymouth, MN 55442
Phone: (651) 756-5400
Keeping your heart healthy with medical technologies that help you and your doctor better manage your health.
Abbott Neuromodulation
8701 Bee Caves Road
Building 2 West
Austin, TX 78746
Phone: (800)-727-7846, option 4
Treating chronic pain and movement disorders by targeting specific areas of the spinal cord and brain.
Abbott Molecular Diagnostics
1300 E Touhy Ave
Des Plaines, IL 60018
Phone: (800) 553-7042
Testing options that provide more accurate means of detection and monitoring of diseases around the world.
Abbott Diabetes Care
1360 South Loop Road
Alameda, CA 94502
Phone: (855) 632-8658
Giving people with diabetes the freedom to monitor and track their glucose levels continuously without the pain of fingersticks.
Abbott Core Laboratory Systems
675 N. Field Drive
Lake Forest, IL 60045
Phone: (224) 667-6100
Helping transform healthcare around the world through the use of analytics, informatics and automation for clinical chemistry.
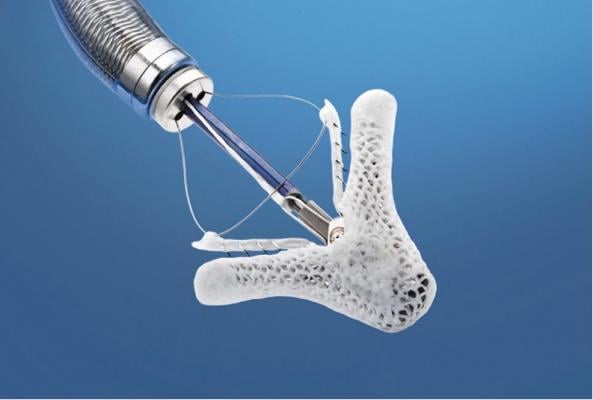
FDA Advisory Committee Supports MitraClip for Patients With Significant Mitral Regurgitation Who Are Too High Risk for Surgery
[caption id="" align="aligncenter" width="593"] FDA Advisory Committee Supports MitraClip for Patients With Significant Mitral Regurgitation Who Are Too High Risk for Surgery[/caption]
Abbott announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has voted by majority (Yes: 5, No: 3) that the benefits of treatment with the MitraClip device outweigh its risks in patients with significant symptomatic mitral regurgitation (MR) who have been determined by a cardiac surgeon to be too high risk for open mitral valve surgery and in whom existing co-morbidities would not preclude the expected benefit from correction of the MR.
Abbott's MitraClip device, which received CE mark in 2008 and is commercially available in Europe and other international markets, is an investigational device in the United States. The device is delivered to the heart through the femoral vein, a blood vessel in the leg, and is designed to reduce MR by clipping together a portion of the leaflets of the mitral valve to allow the heart to more efficiently pump blood.
"We appreciate the FDA's dedication of time and resources to convene this advisory panel for MitraClip and for its review of this new and novel technology for high surgical risk patients suffering from the debilitating symptoms of significant mitral regurgitation," said Charles A. Simonton, M.D., FACC, FSCAI, divisional vice president, Medical Affairs, and chief medical officer, Abbott Vascular. "
On the separate question of whether there is reasonable assurance the device is safe, the panel voted Yes: 8, No: 0. On the question of whether there is reasonable assurance of efficacy, the panel voted Yes: 4, No: 5. The FDA will take into account the panel's advice in making its decision on whether to approve the MitraClip for the treatment of significant MR in the United States. The company expects a decision later this year.
The committee's recommendation followed a review of data from a large and growing body of clinical evidence (EVEREST II, EVEREST II High Risk and REALISM) in which the MitraClip therapy demonstrated positive and consistent results for high surgical risk patients suffering from the debilitating symptoms of significant MR, including a safe procedure, reduction in MR, reverse left ventricular remodeling, improvement in heart failure symptoms, improvements in quality of lifeand reduced rates of re-hospitalization.
For more information: www.abbott.com